Templates
Preview

Description
- Use these templates whenever you have active participants in your study.
- If your study is a record study, these templates are not required.
- Only delete the information that is highlighted in gray.
- It is mandatory to use both versions of this template (spanish and english) for audit purposes.
- If your protocol is Exempt, please delete the identifying fields and use the anonymous fields.
- If your protocol is Expedited or Full Board, please delete the anonymous fields (if your protocol is not anonymous) and use the identifying fields.
Preview

Description
- This template is used when you are recruiting participants that are minors. (more than 7 years old and less than 21 years old)
- It is mandatory to use both versions of this template (spanish and english) for audit purposes.
- Recruiting minors to your investigation will automatically classify your protocol as Full Board Review.
Preview
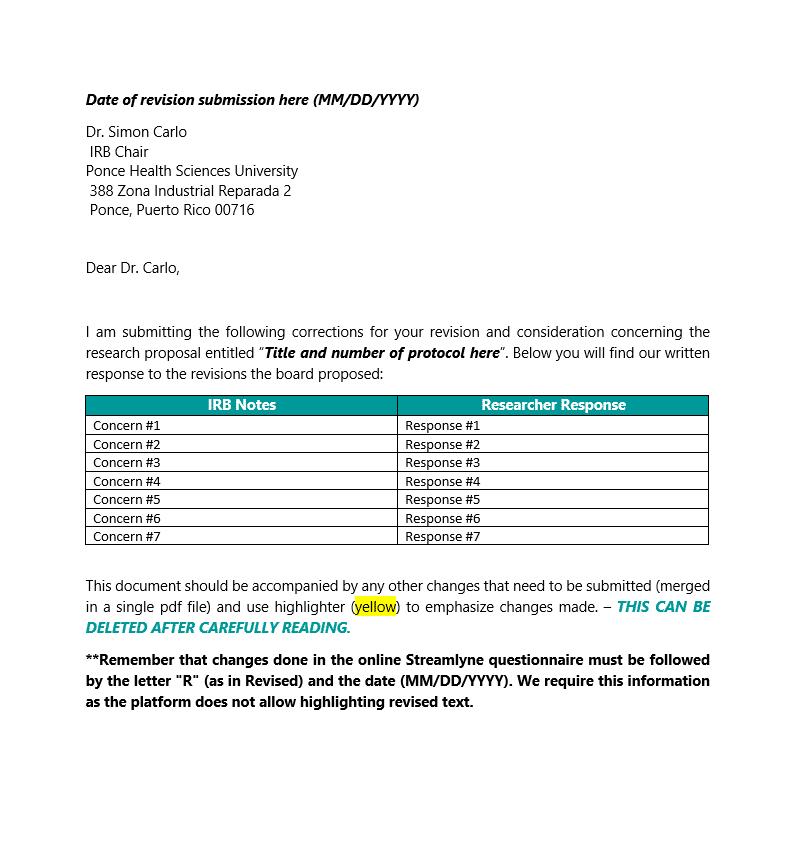
Description
- Use this template to answer IRB correspondence. (Minor Revisions or Substantive Revisions)
- DO NOT use this template to answer a Return to PI. In these cases, you will just correct your protocol and reupload it.
Preview

Description
- This template is used for when you are making an amendment or renewing your protocol.